Symbol |
Name |
# of p+ |
# of no |
# of e- |
atomic # |
atomic mass |
|---|---|---|---|---|---|---|
| Al+3 | aluminum ion | 13 | 14 | 10 | 13 | 27 |
| O-2 | oxide ion | 8 | 8 | 10 | 8 | 16 |
| Co+2 | cobalt (II) ion | 27 | 32 | 25 | 27 | 59 |
| F-1 | fluoride ion | 9 | 10 | 10 | 9 | 19 |
| Hg | mercury | 80 | 121 | 80 | 80 | 201 |
e) silicon tetrafluoride f) nitrous acid g) iron (III) nitrate h) nitrogen dioxide i) manganese (II) chromate
j) cobalt (II) phosphate
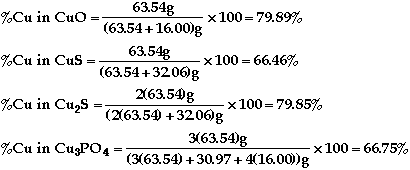
so CuO has the highest %Cu (barely)