Determining the Formula of a Compound
- Introduction/Background:Follow the lab format handout.
- Hypothesis: State what you think the formula will be and why.
- Procedure: Copy this into your lab book.
- Materials: Sodium hydroxide solution, cobalt (II) chloride solution, 2 single row microplates, phenolphthalein solution, micropipets, and goggles.
- Procedure:
- In one of the microplates, add 1 drop CoCl2 solution to the 1st well, 2 drops to the 2nd well, continuing until there are 11 drops in well #11. The last well is empty.
- Add 1 drop phenolphthalein solution to each well.
- Add 11 drops NaOH solution to the 1st well, 10 to the 2nd well, continuing until there is 1 drop in well #11.
- Use a plastic toothpick to mix each well. Wipe the toothpick with a tissue after each well so you do not contaminate the next well.
- Repeat steps 1-4 with the second microplate.
- Record the number of drops of CoCl2 solution and NaOH solution in each well for both trials.
- Observe and record the color of each well as well as the level of the precipitate.
- Note which well is the first one to remain magenta (dark pink). The wells after it will be magenta, but note the first one.
- Clean the microplates by rinsing several times with water. Any residual stain can be wiped out with a cotton swab.
4. Data: Your data table for each trial could look like:
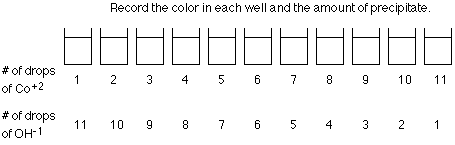
5. Data Analysis:
- For each trial, calculate the ratio of the drops of
OH-1 needed to react with Co+2.
Ratio = (OH-1 drops)/(Co+2 drops) - Explain the meaning of the drop ratio calculated in step A.
- Write the two formulas of cobalt hydroxide using the ratios calculated in step A.
- Look up the possible charges for cobalt. Are your formulas possible?
- If you could choose only one trial, which would you choose? Why?
- After you added the required drops of Co+2 and OH-1 to the wells, did you notice any other colors in the wells? What were they? How do you account for these colors?
- Cobalt also has a +3 charge. If you had used it instead of the +2, how would the results have differed? Give an example to explain.
6. Evaluation: as usual.
7. Conclusion: as usual.
Return to Chemistry 20